1) Electrocatalysis
The ever-growing energy demand and the imminent threat of climate change are two major challenges facing our planet. The global energy consumption reached about 162000 TWh in 2019, along with a corresponding 36 Gt of carbon dioxide emissions. The main portion (~84%) of this energy demand is still supplied by fossil fuels (coal, oil, and gas). Hence, there is an urgent need to replace fossil fuels with clean and renewable energy sources which can diminish carbon dioxide emissions. A promising strategy to achieve this goal is to develop electrochemical processes that can transform abundant molecules (water, nitrogen, carbon dioxide) into products with higher values (hydrogen, hydrocarbons, ammonia). Here is where electrocatalysts can play a pivotal role by facilitating the rate and improving the efficiency and selectivity of the aforementioned electrochemical transformations.
One of the most widely studied electrochemical processes is electrochemical water splitting. It has shown great promise for the efficient and clean production of molecular hydrogen, an environmentally friendly and high-energy density fuel for future applications. Another prominent electrochemical transformation is the electroreduction of CO2, which can yield various products, including formic acid, methanol, carbon monoxide, methane, and ethylene.
Our lab aims to develop efficient and low-cost electrocatalysts for the electrochemical water splitting and electroreduction of carbon dioxide while gaining more insights into the underlying mechanisms of these two fundamental electrochemical processes.
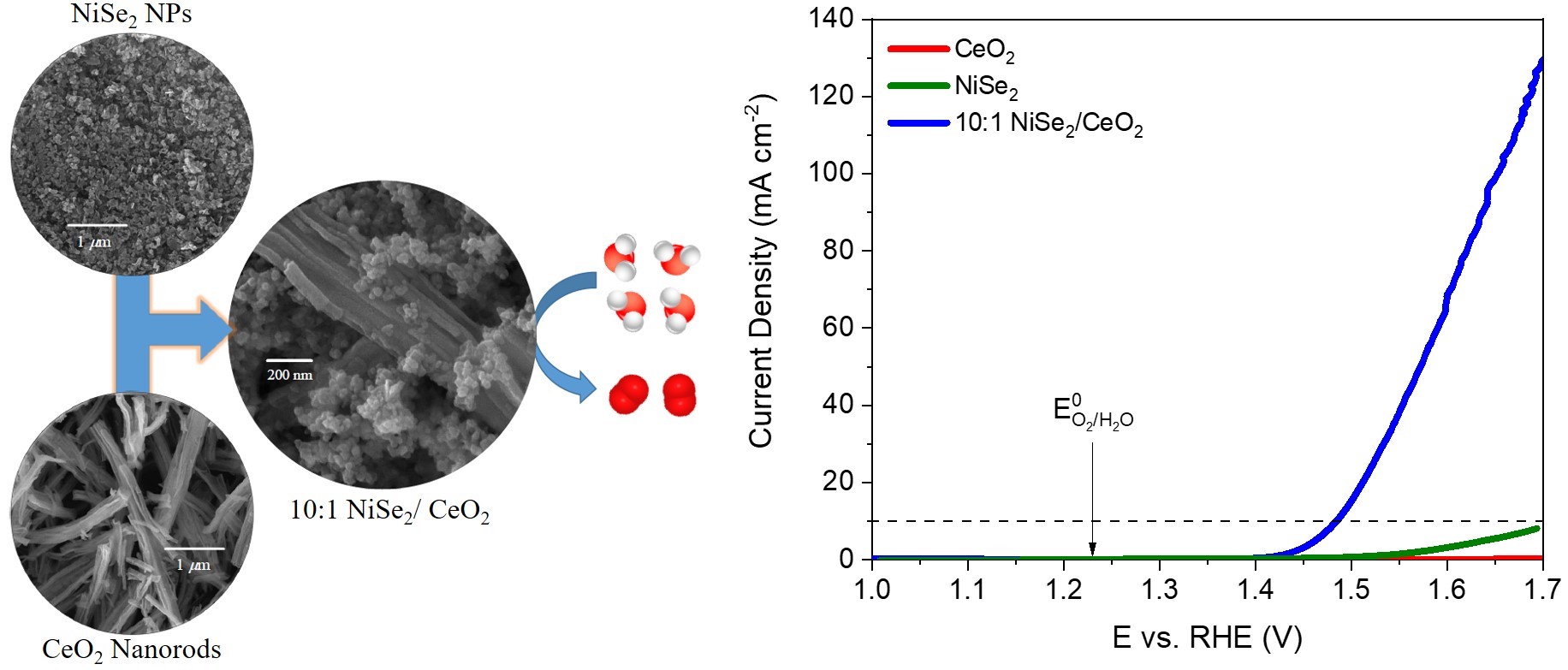 NiSe2/CeO2 nanocomposite is an efficient electrocatalyst for the oxygen evolution reaction (OER). |
2) Interfacial Charge Transfer in Self-Assembled Monolayers
In the past few decades, an enormous amount of research has been done on the charge transfer (CT) process at the metal/molecule interface. Besides the rich science in this area, the studies are motivated by several important technological applications such as sensors, solar cells, light-emitting diodes, electrocatalysis, and molecular electronics. Redox moieties that are immobilized on the surface of metals as a part of a well-defined, organized structure (i.e., a self-assembled monolayer or SAM) are good candidates for investigating interfacial CT reactions. In such structures, the monolayer mediates the CT between the metallic electrode and the redox moiety in a metal-bridge-redox couple structure equivalent to donor-spacer-acceptor configuration extensively studied in homogenous systems and biological structures. Moreover, connecting the redox couple to the surface via a molecular tether would allow putting the couple at a precisely controlled (and variable) distance from the surface while changing the chemical structure of the bridge. This structure would also eliminate complications produced due to the redox couple's convection, diffusion, and adsorption.
Our goal is to explore the impact of molecular structure on the kinetics of interfacial CT in SAMs and to elucidate the CT mechanism.
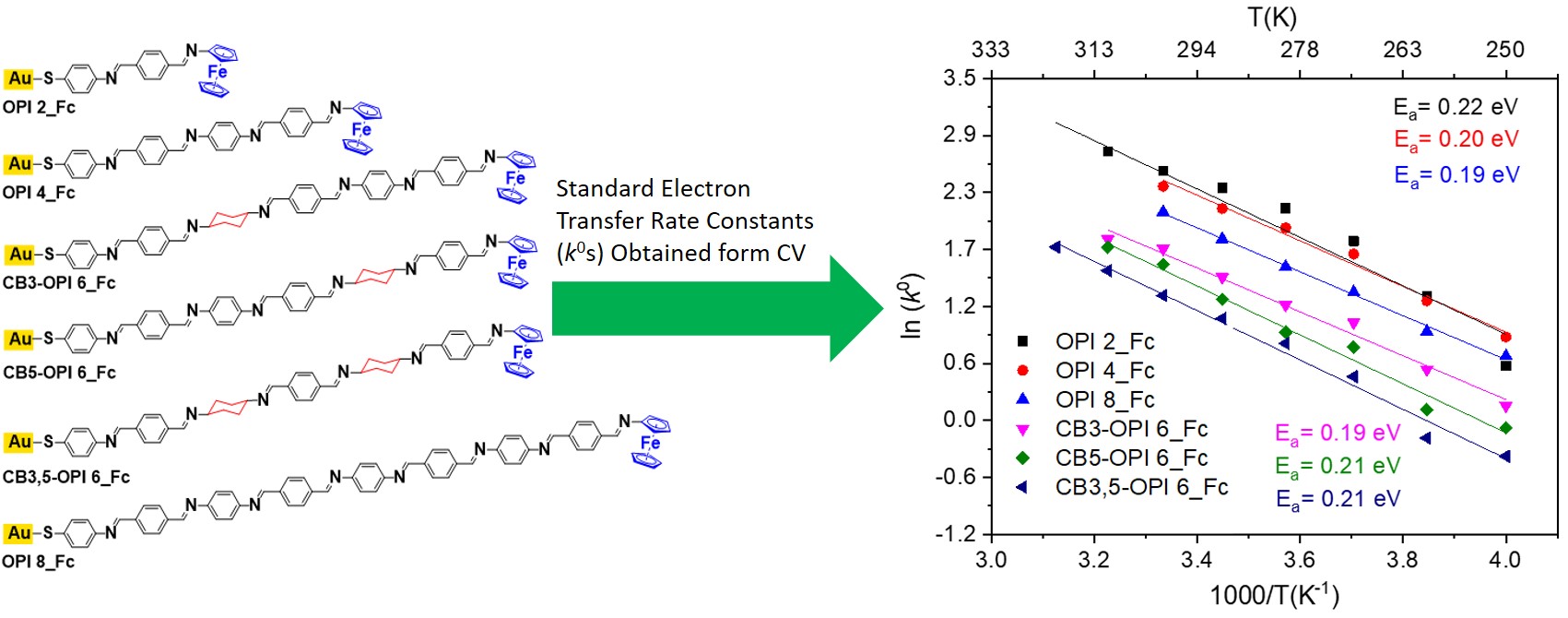 The standard charge transfer rate constant (k0) in ferrocene-terminated oligophenyleneimine (OPI_Fc) self-assembled monolayers exhibit Arrhenius-type temperature dependence. |
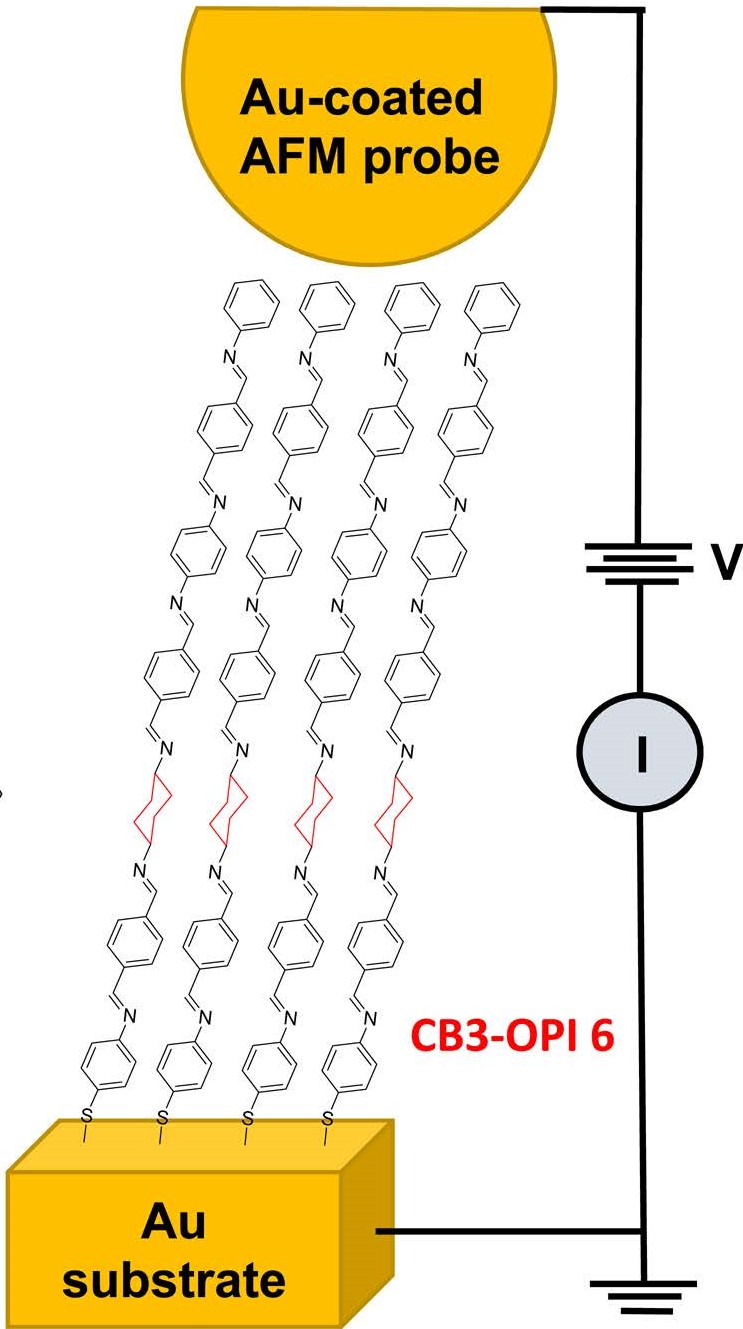
3) Conducting Probe Atomic Force Microscopy (CP-AFM)
In the past few decades, an enormous amount of research has been done on the charge transfer (CT) process at the metal/molecule interface. Besides the rich science in this area, the studies are motivated by several important technological applications such as sensors, solar cells, light-emitting diodes, electrocatalysis, and molecular electronics. Redox moieties that are immobilized on the surface of metals as a part of a well-defined, organized structure (i.e., a self-assembled monolayer or SAM) are good candidates for investigating interfacial CT reactions. In such structures, the monolayer mediates the CT between the metallic electrode and the redox moiety in a metal-bridge-redox couple structure equivalent to donor-spacer-acceptor configuration extensively studied in homogenous systems and biological structures. Moreover, connecting the redox couple to the surface via a molecular tether would allow putting the couple at a precisely controlled (and variable) distance from the surface while changing the chemical structure of the bridge. This structure would also eliminate complications produced due to the redox couple's convection, diffusion, and adsorption.
Our goal is to explore the impact of molecular structure on the kinetics of interfacial CT in SAMs and to elucidate the CT mechanism.
|
|